Which of the following is NOT a type of silicate structure?
Need a Hint?
A) loop silicate
B) double chain silicate
C) single-chain silicate
D) sheet silicate
2
Which of the following are true for a mineral.
Need a Hint?
A) It is inorganic.
B) It is a solid crystalline substance.
C) It is naturally occurring.
D) All of these are properties of a mineral.
3
The silicon-oxygen tetrahedron is:
Need a Hint?
A) the building block of the silicate minerals.
B) composed of 4 oxygen atoms surrounding 1 silicon atom.
C) composed of the two most abundant elements on Earth.
D) all of these
4
The mineral, olivine, is an example of:
Need a Hint?
A) a double-chain silicate structure
B) a sheet silicate structure
C) a framework silicate structure
D) an isolated silicate structure
5
What type of bonding occurs between sodium (Na+2) and chlorine (Cl-2) in the mineral halite?
Need a Hint?
A) covalent
B) weak electrostatic bonds
C) metallic
D) ionic
6
Coal is a rock and not a mineral because:
Need a Hint?
A) it contains only one element: carbon
B) it is made of partially decomposed organic matter
C) it is too soft to be a mineral
D) it does not have cleavage
7
Atoms with either a positive or negative charge are called:
Need a Hint?
A) radioactive
B) elements
C) ions
D) isotopes
8
The type of bonding where electrons in the outer shell of an atom are shared with the adjacent atom:
Need a Hint?
A) ionic
B) weak electrostatic bonds
C) metallic
D) covalent
9
A unit of matter which cannot be broken down into other substances by ordinary chemical methods is:
Need a Hint?
A) an element
B) an atom
C) a molecule
D) a mineral
10
Which of the following is true of a single silica tetrahedron?
Need a Hint?
A) It has a net negative charge.
B) It has one silicon atom and four oxygen atoms.
C) The atoms of the tetrahedron are strongly bonded together.
D) All of these are true of a single silica tetrahedron.
11
The ability of a mineral to break along preferred planes is called:
Need a Hint?
A) brittleness
B) hardness
C) fracture
D) cleavage
12
Which of the following are minerals?
Need a Hint?
A) all of the choices below are minerals.
B) calcite
C) quartz
D) salt
13
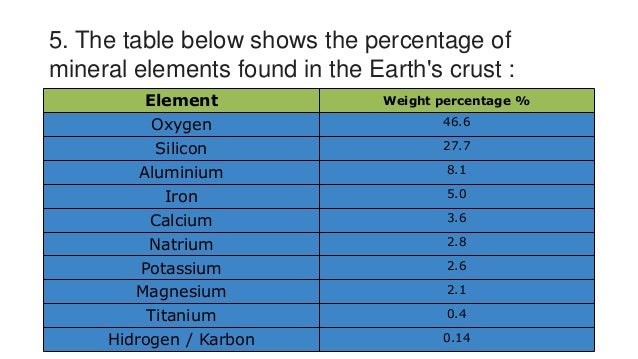
Oxygen accounts for what percent by weight (approximately) of all matter in Earth's crust?
Need a Hint?
A) 12%
B) 24%
C) 36%
D) 47%
14
Amphibole is:
Need a Hint?
A) a sheet silicate
B) a single chain silicate
C) a framework silicate
D) a double chain silicate
15
Protons are sub-atomic particles:
Need a Hint?
A) which orbit around the nucleus of the atom.
B) with a positive electrical charge.
C) with a negative electrical charge.
D) with variable atomic mass.
16
An ion with a surplus of electrons is:
Need a Hint?
A) an isotope.
B) neutral.
C) positively charged.
D) negatively charged.
17
Stable isotopes are elements that:
Need a Hint?
A) have variable numbers of protons.
B) do not lose protons and neutrons over time.
C) are radioactive.
D) none of these.
18
Which of the following mineral pairs are not polymorphs??
Need a Hint?
A) calcite and aragonite
B) diamond and graphite
C) fosterite and fayalite
19
The most common group of minerals are the:
Need a Hint?
A) sulfides
B) silicates
C) oxides
D) carbonates
20
Atoms become ions when they:
Need a Hint?
A) gain or lose mass.
B) gain or lose neutrons.
C) gain or lose protons.
D) gain or lose electrons.
KEY: A = 1.9.12
B = 6.15.17.19
C = 7.18
D = 2.3.4.5.8.10.11.1314.16.20
1
Diamonds are the hardest natural mineral.
Need a Hint?
A) TRUE
B) FALSE
2
Gypsum is the softest naturally occurring substance on Earth.
Need a Hint?
A) TRUE
B) FALSE
3
Diamonds are found only in South Africa.
Need a Hint?
A) TRUE
B) FALSE
4
Minerals are composed of compounds.
Need a Hint?
A) TRUE
B) FALSE
5
All silicate minerals are composed of silicon-oxygen tetrahedra arranged in various ways.
Need a Hint?
A) TRUE
B) FALSE
6
By definition, water is a mineral.
Need a Hint?
A) TRUE
B) FALSE
7
By definition, petroleum is a mineral.
Need a Hint?
A) TRUE
B) FALSE
8
By definition, coal is mineral.
Need a Hint?
A) TRUE
B) FALSE
9
Diamonds are formed by carbon subjected to very high pressure deep inside the Earth.
Need a Hint?
A) TRUE
B) FALSE
10
Atoms may gain or lose neutrons.
Need a Hint?
A) TRUE
B) FALSE
11
Atoms may gain or lose electrons.
Need a Hint?
A) TRUE
B) FALSE
12
Eight elements comprise 98.5% of Earth's crust.
Need a Hint?
A) TRUE
B) FALSE
13
The most abundant element in Earth's crust is carbon.
Need a Hint?
A) TRUE
B) FALSE
14
The most abundant element in Earth's crust is oxygen.
Need a Hint?
A) TRUE
B) FALSE
15
The most abundant element in Earth's crust is hydrogen.
Need a Hint?
A) TRUE
B) FALSE
16
All compounds have a crystalline structure.
Need a Hint?
A) TRUE
B) FALSE
17
Common table salt is known to geologists as the mineral, halite.
Need a Hint?
A) TRUE
B) FALSE
18
Some minerals can be scratched with your fingernail.
Need a Hint?
A) TRUE
B) FALSE
19
All minerals display a physical property known as hardness.
Need a Hint?
A) TRUE
B) FALSE
20
All minerals display a physical property known as fracture.
Need a Hint?
A) TRUE
B) FALSE
KEY: TFFFT FFFTT TTFTF FTTTT
Walang komento:
Mag-post ng isang Komento